
Likewise, if bonding with another Sulfur atom, there would be a polarity of zero and no one atom would attract the electrons because the atoms are of the same radius and size. In addition, if Sulfur was to be in a covalent bond, depending on the electronegativity of the other element, the electrons would probably be closer to the nucleus of Sulfur. This means that if in a ionic bond Sulfur has the ability to take the electrons away from the metal. Sulfur is a nonmetal and has a high electronegativity of 2.58. In addition, nonmetals have higher electronegativities so when they are bonding with a metal, they have the ability to take the electron completely, not sharing it like a covalent bond but becoming an ionic bond. If the covalent bond is between two identical atoms, with the same electronegativity, then there will be no pull and the bond will be non-polar covalent. In this case, it would make the bond, polar covalent. If this is true then the shared bonding pair of electrons in the covalent bond, will be closer to the nucleus of that elemental atom. When in a covalent bond, one of the elements may have a higher electronegativity. There are many different types of bonds that are related to electronegativity. However, as you move across the periodic table you see that the electronegativity increases, because the radius is decreasing and the attraction is stronger between the electrons and the nucleus.
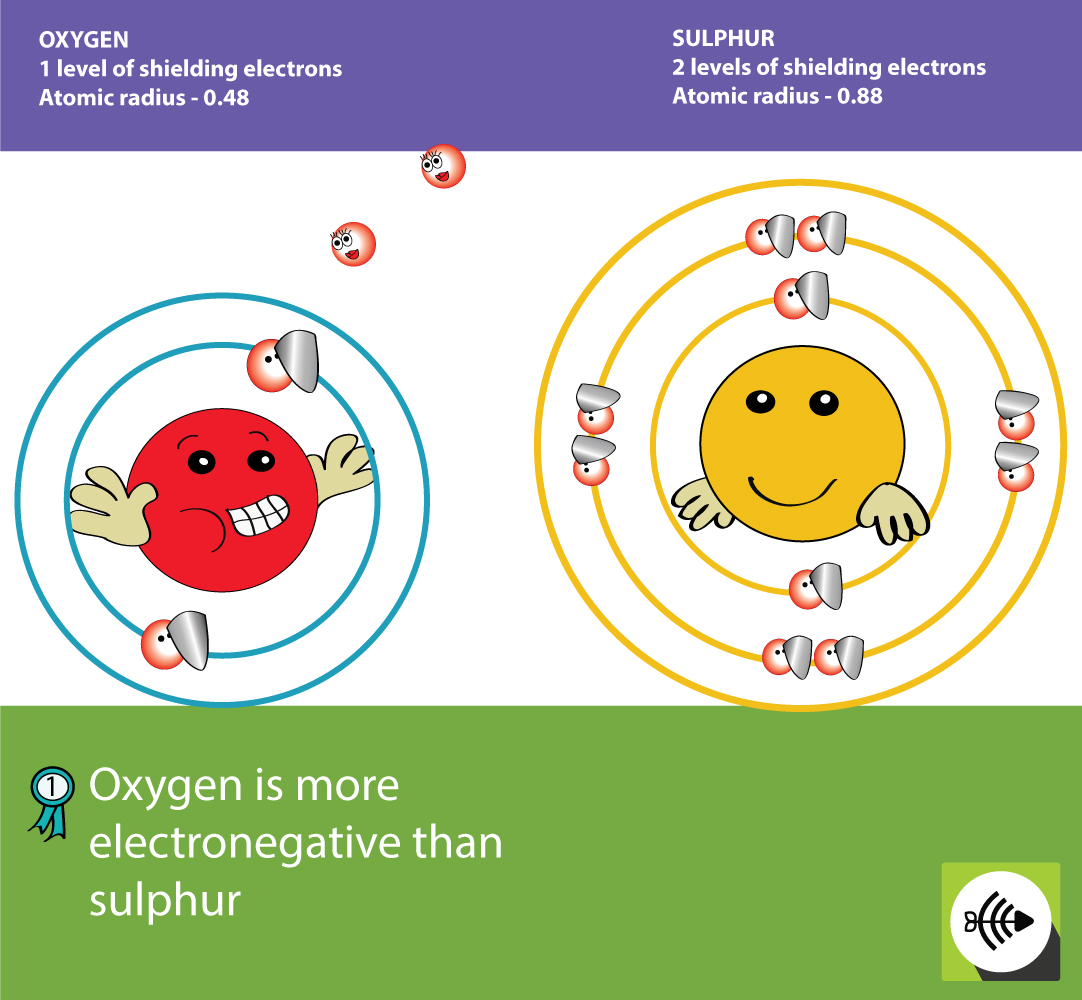
Because sulfur is farther right than phosphorous is, it has a a smaller radius. This is because atomic radius tends to decrease as you go left to right across the periodic table. If the radius increases then the attraction between the nucleus also decreases and the strength of that atom to attract a bonding pair of electrons will decrease, thus having low electronegativity. the radius of sulfur is smaller than the radius of phosphorous. As you go down the periodic table groups, the electronegativity decreases because electronegativity is directly related to atomic radius, more than atomic number. Electronegativity is the ability for an atom to attract a bonding pair of electrons. This is a factor when it comes to bonding.


 0 kommentar(er)
0 kommentar(er)
